Introduction
Each Vascular Research Tools (VRT) or Vascular Tools (VT) license is locked to a single computer after the installation and license activation.
The license may be transferred from one computer to another. License transfer is supported as long as the total number of installations does not exceed the total number of licenses purchased.
There are 2 simple steps to transfer a license from computer A to computer B:
- De-activate and un-install the license on computer A.
- Install and activate the license on computer B.
The deactivation process is the same across versions (v.5.6.5 to current).
The activation process is different for older versions (v. 6.12 and older) and newer versions (v6.13 and later) - see below.
Details of the deactivation and activation steps follow. Contact MIA if you have an earlier version.
De-activate and Un-install the License
- Log on the computer with an active VRT or VT installation to perform the following license transfer. Make sure you have the sufficient administrative privilege. For v6.13 or later, the computer must connect to the internet.
- Run the Brachial/Carotid Analyzer (for Research), go to the Help menu and then the Licensing.
- You will see the licensing screen similar to this:
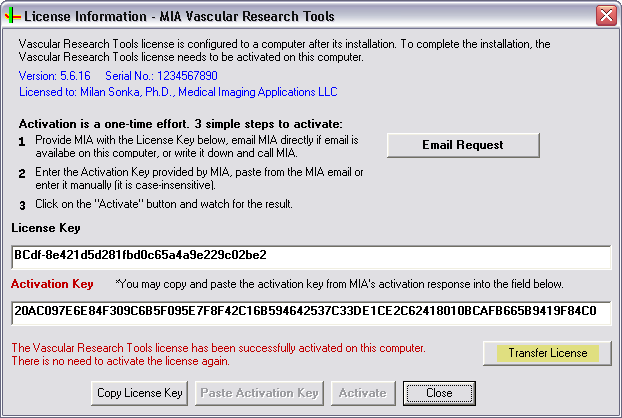
- Click on the Transfer License button. Note: earlier versions may not have this Transfer License button, email MIA at email address: support@mia-llc.com if this is the case and skip the steps below.
- You will see the license-deactivation window similar to this:
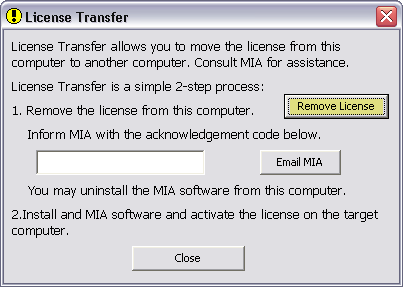
- Click on the Remove License button, and confirm the removal action.
- Once confirmed and the license is de-activated, there will be an acknowledgement code displayed similar to this:
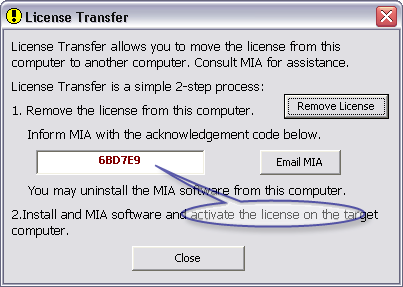
- For versions 6.13 and later - skip this step.
For versions 6.12 and older - Inform MIA this 6-letter acknowledgement code. If the computer has email access, click on the Email MIA button. Otherwise write down the code and inform MIA separately by emailing MIA at email address: support@mia-llc.com
- You may un-install the MIA Vascular Research Tools software on this computer.
Install and Activate the License on the New Computer for versions 6.13 and later
- Log on to the computer on which the VRT/VT license is being transferred, make sure you have the sufficient administrative privilege and Internet connectivity.
- Install the VRT/VT software using the same installation media you used for installing on the previous computer.
- Activate the VRT/VT license the same way as previously - the activation process is automatic, activation will communicate with MIA license server - activation on a new computer is only possible if the license was already deactivated on the old computer.
Install and Activate the License on the New Computer for versions 5.6.5 - 6.12
- Log on to the computer on which the VRT/VT license is being transferred, make sure you have the sufficient administrative privilege.
- Install the VRT/VT software using the same installation media you used for installing on the previous computer.
- Activate the VRT/VT license by emailing the license code to MIA and obtaining a new activation code from MIA.
|


